Recent Blog Posts
Shoulder Injuries Following Vaccination (SIRVA)
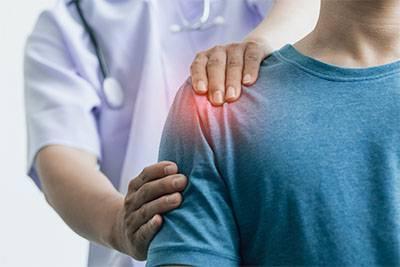 Shoulder soreness following vaccination is commonly experienced, but not all post- vaccination shoulder pain, particularly if severe, is normal. In the mid 2000’s, two physicians published an article about severe, chronic shoulder pain triggered in two patients by their recent vaccinations. A few years later, another piece of medical literature used the acronym SIRVA, or Shoulder Injury Related to Vaccine Administration, for the first time to refer to these post-vaccination injuries.
Shoulder soreness following vaccination is commonly experienced, but not all post- vaccination shoulder pain, particularly if severe, is normal. In the mid 2000’s, two physicians published an article about severe, chronic shoulder pain triggered in two patients by their recent vaccinations. A few years later, another piece of medical literature used the acronym SIRVA, or Shoulder Injury Related to Vaccine Administration, for the first time to refer to these post-vaccination injuries.
SIRVA refers to a set of medical conditions impacting a shoulder following vaccination and can include bursitis, tendinitis, rotator cuff tearing, and adhesive capsulitis, among others. While some individuals experience mild symptoms with a good recovery within a few months, for others, SIRVA injuries may be persistent and require medical interventions like physical therapy, cortisone injections, or even surgery. While the exact mechanism of SIRVA injures is unknown, it is suspected to be caused by improper vaccination administration technique and/or by the inflammatory response triggered by vaccination.
An Association Between Guillain-Barre syndrome and the Seasonal Flu Vaccine
 Guillain-Barre syndrome, also known as GBS, is a rare condition in which an individual’s own immune system attacks their nerve endings. While there are different subvariants of GBS, the hallmark symptoms of the condition include weakness, numbness, tingling and for some individuals, paralysis. Fatigue is also often reported. More information about GBS can be found here.
Guillain-Barre syndrome, also known as GBS, is a rare condition in which an individual’s own immune system attacks their nerve endings. While there are different subvariants of GBS, the hallmark symptoms of the condition include weakness, numbness, tingling and for some individuals, paralysis. Fatigue is also often reported. More information about GBS can be found here.
The association between GBS and vaccination was initially suspected after a large-scale influenza vaccination effort in 1976 revealed an increased signal of GBS among the vaccinated population. Subsequent studies confirmed an association between GBS and the 1976 flu vaccination efforts.
While vaccine induced GBS is still considered a rare event, the association with flu vaccines is well established. As a result of this association, GBS is listed as a Table injury in the Vaccine Injury Compensation Program (VICP). As a Table injury, if a person who receives the vaccine (referred to as the “petitioner”) can establish that they developed GBS within 3-42 days of receiving the vaccination, then the injured person is likely to receive compensation.
Covid-19 Vaccine Injuries and the Countermeasures Program
 As more Americans undergo vaccination for Covid-19, it is inevitable that rare adverse events will occur.
As more Americans undergo vaccination for Covid-19, it is inevitable that rare adverse events will occur.
While we represent individuals injured by vaccines covered under the Vaccine Injury Compensation Program (VICP), vaccine injuries stemming from a Covid-19 vaccination are, at present, not handled through the VICP.
Instead, Covid-19 vaccine injuries are litigated through the Countermeasures Injury Compensation Program (CICP).
During periods of national emergency, including medical emergencies such as during the Ebola and Zika epidemics, the Secretary of Health and Human Services (HHS) has the power to recommend a countermeasure, such as a vaccine or medication, with the aim of preventing or stopping those events. When HHS recommends such a countermeasure, any injury stemming from that covered countermeasure can be compensated through the CICP.
As Covid-19 was declared a national medical emergency in March 2020, at this time, Covid-19 vaccine injuries are only compensable through the CICP.
HHS Withdraws Rule Change Impacting Shoulder Injuries in the Vaccine Compensation Program
 On April 22, 2021, Health and Human Services (HHS) published the final rule on the Federal Register withdrawing the proposed rule change previously published on January 21, 2021 which would have changed how cases involving shoulder injuries and vasovagal syncope were handled in the Vaccine Injury Compensation Program (VICP).
On April 22, 2021, Health and Human Services (HHS) published the final rule on the Federal Register withdrawing the proposed rule change previously published on January 21, 2021 which would have changed how cases involving shoulder injuries and vasovagal syncope were handled in the Vaccine Injury Compensation Program (VICP).
In explaining this action, HHS noted that members of the public had expressed concern that the agency’s process while pursuing the proposed rule removing shoulder injuries and vasovagal syndrome from the Vaccine Injury Table was irregular in its haste. HHS also observed that from a public health policy perspective, removing these injuries from the Vaccine Injury Table may dissuade individuals from undergoing vaccinations, which would be counter to the public’s interest in promoting vaccination. You can find HHS’s full rationale for withdrawing the rule here.
Proposed Vaccine Compensation Program Rule Change Impacting Shoulder Injuries Likely to be Withdrawn
 On March 17, 2021, Health and Human Services (HHS) published a notice on the Federal Register alerting the public that the proposed rule change previously published on January 21, 2021 changing how cases involving shoulder injuries and vasovagal syncope are handled in the Vaccine Injury Compensation Program (VICP), is likely to be withdrawn following a thirty day comment period for public response ending on April 16, 2021.
On March 17, 2021, Health and Human Services (HHS) published a notice on the Federal Register alerting the public that the proposed rule change previously published on January 21, 2021 changing how cases involving shoulder injuries and vasovagal syncope are handled in the Vaccine Injury Compensation Program (VICP), is likely to be withdrawn following a thirty day comment period for public response ending on April 16, 2021.
The January 2021 rule by HHS would have removed shoulder and vasovagal injuries from the Vaccine Injury Table, requiring injured parties to pursue these injuries through "causation-in-fact" claims which often require the retention of medical experts and very often, an in-person hearing years down the line before a Special Master (Judge) in Washington D.C. That rule change was initially due to go into effect on February 22, 2021, however, the effective date was pushed back two months until April 23, 2021 following a request by the Biden administration for time to review all administrative actions that occurred during the sunset of the previous administration.
Vaccine Program Rule Change Affecting Shoulder Injuries Delayed For Now
 On February 23, 2021, Health and Human Services (HHS) published a rule on the Federal Register that effectively gives individuals that have experienced a shoulder injury or vasovagal syncope following vaccination, an additional two months, until April 23, 2021, to file a vaccine injury claim through the federal Vaccine Injury Compensation Program (VICP).
On February 23, 2021, Health and Human Services (HHS) published a rule on the Federal Register that effectively gives individuals that have experienced a shoulder injury or vasovagal syncope following vaccination, an additional two months, until April 23, 2021, to file a vaccine injury claim through the federal Vaccine Injury Compensation Program (VICP).
Originally, the rule which would change the way these injuries are handled within the VICP, making it more difficult for many vaccine injured individuals to pursue their claims, was due to take effect on February 22, 2021. The two month pause in the rule’s effective date was announced by HHS due to a request by the Biden administration for time to review all administrative actions that occurred during the sunset of the previous administration.
We have previously covered the rule changes, and the likely outcomes for vaccine injured individuals, in prior blog posts which you can find here and here.
Upcoming Changes To The Vaccine Injury Program Draws Attention Of National Press
 On February 4, 2021, USA Today published an article about the upcoming removal of Shoulder Injury Related to Vaccine Administration (“SIRVA”) from the vaccine injury table. We discussed this rule change, which goes into effect on February 22, 2021, in a previous blog post. You can find our blog discussing that issue here.
On February 4, 2021, USA Today published an article about the upcoming removal of Shoulder Injury Related to Vaccine Administration (“SIRVA”) from the vaccine injury table. We discussed this rule change, which goes into effect on February 22, 2021, in a previous blog post. You can find our blog discussing that issue here.
The USA Today article discusses not only the rule change impacting SIRVA, but also touches on additional rule changes put in place by Health & Human Services (HHS) at the same time, all pushed through at the very end of the Trump administration with at best, minimal support, (chiefly from HHS). There was substantial pushback from players in the vaccine injury program, including the Advisory Commission on Childhood Vaccines (ACCV), the Vaccine Injured Petitioners Bar Association, members of the general public, physicians, pharmaceutical chains, etc., and individuals that have taken their SIRVA cases through the vaccine injury program.
NPRM

On January 21, 2021, Health and Human Services (HHS) published a final rule in the Federal Register that changes a key part of the Vaccine Injury Compensation Program (VICP) addressing how shoulder injuries following vaccination, as well as vasovagal syncope, are dealt with in the Program.
While vaccine injuries are considered to be rare, shoulder injuries following vaccination are among the most common vaccine adverse events and so in 2017, the Vaccine Injury Table was amended to add Shoulder-Injury-Related-to-Vaccine-Administration (SIRVA) to the Table.
By adding SIRVA to the Table, these claims were intended to be resolved in a more timely and efficient manner compared to causation-in-fact vaccine injury claims which often require the retention of medical experts as part of a years long litigative process culminating in an in-person hearing before a Special Master in Washington D.C.
Unfortunately, the new rule, which goes into effect on February 22, 2021, removes both SIRVA, and vasovagal syncope, from the Table.
An Overview of Vaccine Safety During the Time of Covid-19
 As the clamor grows for an effective Covid-19 vaccine, questions regarding vaccine safety have been foremost in the minds of the public. A recently published article in the pre-eminent journal, Science, by lead author, David M. Knipe, provides an overview into the vaccine safety regulatory framework in place in the United States and discusses how that framework came-to-be over the last several decades.
As the clamor grows for an effective Covid-19 vaccine, questions regarding vaccine safety have been foremost in the minds of the public. A recently published article in the pre-eminent journal, Science, by lead author, David M. Knipe, provides an overview into the vaccine safety regulatory framework in place in the United States and discusses how that framework came-to-be over the last several decades.
Among the common vaccine safety measures discussed by the authors are quality control checks to ensure that each batch of vaccine meets strict formulation standards and monitoring adverse events both before and after licensing to determine whether additional study, or alternate action, is needed. The article also discusses the Food and Drug Administration’s (“FDA”) specific guidance to pharmaceutical companies developing potential Covid-19 vaccines, including recommendations for post-licensure reporting and post-licensure studies in light of the likelihood for emergency-use approval of Covid-19 vaccines.
Avoiding SIRVA injury During the 2017-2018 Flu Season
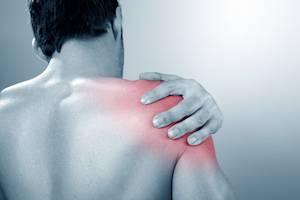 Last week, makers of the influenza vaccine began shipping their first doses of flu vaccine for the 2017 – 2018 flu season to healthcare providers, pharmacies, and immunizers. People will be able to begin getting immunized for the upcoming flu season beginning in late September.
Last week, makers of the influenza vaccine began shipping their first doses of flu vaccine for the 2017 – 2018 flu season to healthcare providers, pharmacies, and immunizers. People will be able to begin getting immunized for the upcoming flu season beginning in late September.
There are a small number of people who should take precautions when getting the flu vaccine, and a small number of people who should not get it at all. Suitability for the vaccine is determined by health status, age, and allergies to components of the flu vaccine. You should talk to your health care provider before getting immunized if you are concerned about your suitability for the influenza vaccine.
For the approximately 160 million people who will get the 2017-2018 flu vaccine, it a good time to learn or to review some tips how to protect yourself from Shoulder Injury Related to Vaccine Administration (SIRVA) injury.






